No FDA Guidance on Social Media Leading to Higher Enforcement?
April 4th, 2013 // 2:23 pm @ jmpickett
Mark Senak, author of EyeOnFDA blog, assembled a database of FDA/OPDP Warning and NOV letters spanning the years 2004-present and wrote an informative white paper review of the data (find it here).
April 10 – Avoid the CDRH eCopy Chaos – How to Prepare a Compliant eCopy Submission
“Lacking any sort of formal guidance from the agency [regarding regulation of digital media], the only peek into FDA’s point of view is to examine enforcement patterns,” says Senak. “So I have used the data base to compare enforcement patterns vis a vis digital communications.”
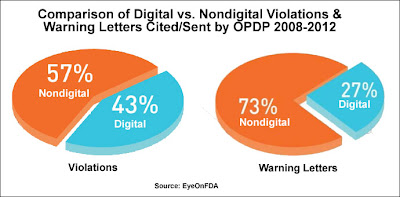
Here are a couple of charts from the white paper showing the breakdown in violations cited and warning letters sent according to type of media – digital vs. nondigital:
On the left is the breakdown of violations. There were a total of 676 violations and 173 letters. “Of all the violations cited,” notes Senak, “a majority involved nondigital media. A comparison shows that 57 percent (n=385) of the vehicles cited for violations were nondigital properties compared to 43 percent (n=290) involving digital-based communications vehicles.”
How many of these violations are serious? Senak looked at the distribution of Warning Letters for that. “Of 45 WLs [Warning Letters] issued by OPDP during this period,” wrote Senak, “only 12 cited digital communications vehicles while 33 were based on nondigital (traditional) communications, meaning that a Warning Letter was almost three times more likely to be based on a traditional media communications vehicle than on a digital one.”
April 10 – Avoid the CDRH eCopy Chaos – How to Prepare a Compliant eCopy Submission
Senak cites an obvious limitation of his data: one cannot say whether or not digital is over or under represented. The reason, claims Senak, is that “we do not know what proportion of communications by industry is divided between digital and non-digital efforts.” Senak contends that “the lack of clarity respecting the rules around Internet and social media use does not appear to translate into a greater pattern of enforcement against digital media.”
I disagree.
I believe we DO have some data that suggests digital is very much OVER REPRESENTED in terms of violations and warning letters. As I pointed out in a previous post (here), only 2% of pharma’s DTC budget is devoted to digital (not counting search advertising). Now I know that Senak’s data also includes professional promotion and is not limited to consumers promotion. Even taking all that into account, it’s impossible that pharma spends 27-43% of its promotional budget/effort on digital channels.
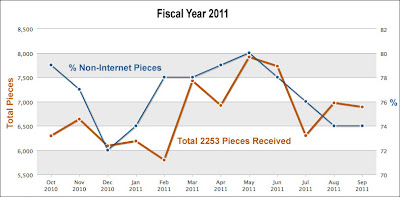
Maybe, however, it’s not the money spent but the number of communications created that we should look at. Let’s look at the “Total 2253 pieces (Transmittal of Advertisements and Promotional Labeling for Drugs and Biologics for Human Use) received” by FDA for fiscal year 2011 (the latest full year of data; here):
The percent of “non-Internet” pieces varies from 72% to 80%, which means the % of Internet pieces is in the 20-28% range (I would say the average was 24% for the year).
So 24% of promotional pieces reviewed by FDA gets 43% of the violations cited and 27% of serious warning letters sent. From this I would have to disagree with Senak and say the lack of clarity respecting the rules around Internet and social media use by pharma DOES appear to translate into a GREATER pattern of enforcement against digital media.
Upcoming Expertbriefings.com Webinars 2013
Check out our latest FDA drug and device news, too
- April 10 – Avoid the CDRH eCopy Chaos – How to Prepare a Compliant eCopy Submission
- April 11 – The Quality Manager Gets Fired, the $100,000 Compliance SNAFU, And 21 Tips and Tricks For Your Next Audit
- April 18 – Why You May Want to Move Your Pharma Company to Kansas – 483 and Warning Letter Trends
- April 21 – $500K on Compliance or $300 Million for Consent Decree? – Essential FDA Compliance Tips for Sr Management
- April 29 – Audit Your Lab Like an Expert FDA Auditor: A Roadmap to Lab Compliance
- April 30 – FDA Hands Out CAPA 483s Like Candy – Avoid Them With a Closed Loop CAPA SystemÂ
- May 1 – Avoiding Warning Letter Disasters With a Strong Contractor Quality Agreement
- May 7 – FDA Recall Chief Update – How to Design a Bulletproof Product Recall Strategy