FDA Data Reveals More Warning Letters to Medical Device Companies
May 27th, 2013 // 3:49 pm @ jmpickett
Latest FDA and cGMP Compliance News
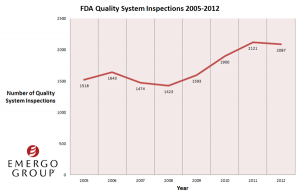
FDA medical device inspectors have been issuing more warning letters since 2005, as an inspection of recent FDA records indicates. The increase in 483s and warning letters is coming along with an increase in QSR inspections in the last 5 years. FDA did 1423 QSR inspections of medical device firms in 2008, and 2121 in 2011. The more QSR inspections FDA does, they are finding more compliance issues, naturally.
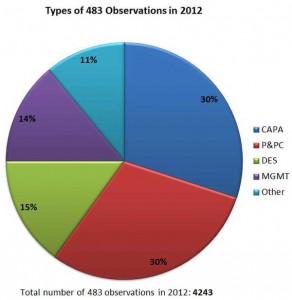
You might be wondering, though what types of noncompliance problems FDA inspectors are seeing the most. And which quality system parts should you pay most attention to? According to the 2012 data that we have seen, you should mostly pay attention to CAPA and Production and Process Control issues. Together these issues counted for 30% of the total 483 observations. Design, document controls and management issues were far behind these concerns.
- May 30 – Audit Your Lab Like an FDAer
- June 12 – Avoid Warning Letter Disasters with a Strong cGMP Quality Agreement
Also, quality system observations have increased a great deal since the agency started to have a more aggressive schedule of medical device inspections. In the last 4 years, warning letter citations have climbed. The highest increase was from 2011-12. However, we do not think that the boost in citations is because there has been a decrease in QS compliance among device companies. We think that there are more inspections so there are more compliance issues being noted.
In our view, the warning letter data for device companies shows that QS citations are increasing. Anyone who works in the device field should expect more QS inspections at least through the conclusion of Obama’s second term. Also, the data shows that FDA really likes to focus on CAPA and PPC procedures in its audits. You want to make sure you are in full compliance with these areas and with 21 CFR Part 820.