New Compounding Pharmacy Scandal in TN
May 29th, 2013 // 1:01 pm @ jmpickett
Latest FDA and cGMP Compliance News
Last month, Tennessee passed a law that eased the restrictions on the state’s compounding pharmacies. Now FDA is looking into seven cases of patients suffering adverse events after they were injected with possibly contaminated drugs that were compounded by a TN pharmacy.
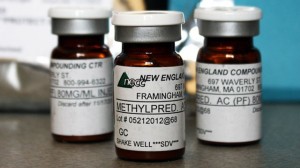
That is why Main Street Family Pharmacy is doing a recall of all of its sterile drug products. Most of them are injectable drugs. It appears that the drugs that are related to the adverse events have methylprednisolone acetate, which is the drug that led to the killer outbreak of fungal meningitis last year.
That outbreak has 740 cases and led to 55 deaths so far. It is the most grave public health crisis in the US in 40 years, and was traced to the New England Compounding Center. That scandal has led FDA to scrutinize drug compounders more closely.
For example, in 2013, FDA started an aggressive effort to do inspections of dozens of drug compounders in many states. There have been some product recalls, too. FDA wants Congress to give them more oversight authority, as well.
- May 30 – Audit Your Lab Like an FDAer
- June 12 – Avoid Warning Letter Disasters with a Strong cGMP Quality Agreement
However, Tennessee last month passed a law to cut down on drug shortages by letting compounders make more drugs and also avoid reliance on compounders out of state. This law also would allow compounders in TN to make/dispense drugs without a specific prescription for a patient. Some experts think this is dangerous.
Records show that Main Street Family Pharmacy shipped product to AL, AR, CA, FL, KY, IL, LA, MS, NM, NC, SC, TN, and TX. So far, patients in NC and IL have infections.
It is not clear how many patients got injected with the tainted drugs, but the TN Department of Health says that the firm had a license as a manufacturer, wholesaler and distributor in 2010.