Why Are Most Americans Ducking Clinical Trials?
June 13th, 2013 // 6:20 pm @ jmpickett
Latest FDA and cGMP Compliance News
Even though there is always more demand for new and better medical treatments, pharmaceutical companies often say that getting people to enroll in clinical trials in the US is quite difficult.
One of the most common reasons is that a large number of our population has good access to needed drugs, which means they are less likely to enroll in trials. Also, it can be hard to find people who can participate without interfering with the drugs they take currently.
Download Your Free Sample – the Latest FDA 483 and Warning Letter Reports!
A new survey has found that there is a limited willingness in the US for consumers to participate in trials. About 73% of 1005 US adults polled said that they would participate in a trial if their doctor recommended it. About 25% said that they would be highly likely and 45% said they would be somewhat likely. This poll was done by Research America, the Association of Clinical Research Organizations, the Clinical Research Forum, the Friends of the National Library of Medicine and the Clinical Trials Transformation Initiative.
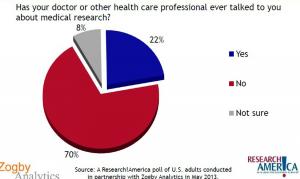
But what is stopping people from signing up? About 70% say their doctors do not talk about medical research. About 80% say that they are aware of trials but most hear about them from online sources or the media. Only about 25% hear about medical trials from doctors. But 60% do want their doctors to tell them about the information.
About 42% said that doctors and other medical care providers should provide more education about clinical trials. Fifty two percent said that they do not participate because they do not know about the clinical trials, but then 52% also say they do not trust the clinical trials themselves.
Some consumers, 51%, think the trials are too risky, and 44% worry about adverse events. Still about 73% said they would participate if it would help people to understand diseases and to find better treatments.
But when it comes to what matters most to people who would consider participating in a clinical trial, the reputation of the people and organization doing the study is very important to 70% of respondents. Also, 68% said that coverage of medical bills due to an injury would be an important factor as well. Sixty one percent said it was very important for the study to improve their health, and 53% were concerned about privacy.
The bottom line here to us is that American consumers are interested in participating in clinical trials, but have several caveats, and need to be better informed about them from their doctors.
What do you think? Why are more patients not participating in clinical trials in the US?